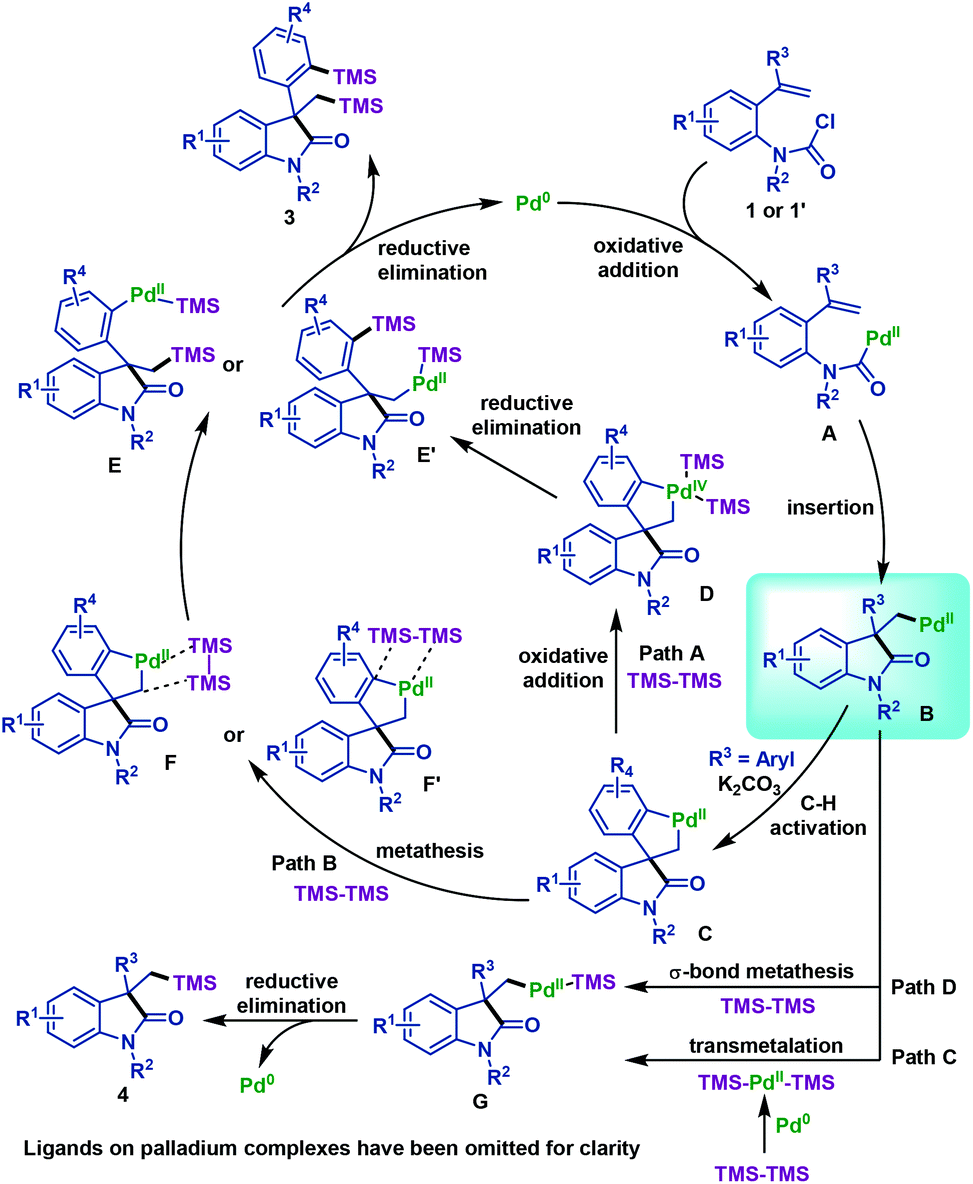
Palladium-catalyzed domino Heck-disilylation and Heck-monosilylation of alkene-tethered carbamoyl chlorides: synthesis of versatile silylated oxindoles - Organic Chemistry Frontiers (RSC Publishing)
Palladium-Catalyzed Synthesis of Dibenzosilepin Derivatives via 1,n- Palladium Migration Coupled with anti-Carbopalladation of Al
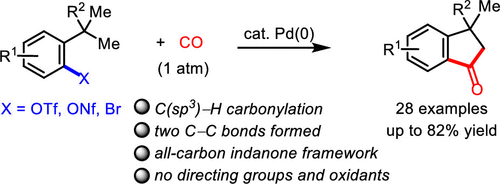
NHC Ligand-Enabled, Palladium-Catalyzed Non-Directed C(sp3)–H Carbonylation To Access Indanone Cores,ACS Catalysis - X-MOL

Stereoselective Heterocycle Synthesis via Alkene Difunctionalization - Bulky Phosphine Ligands Enable Pd-Catalyzed Arylhalogenation, Arylcyanation and Diarylation | David A. Petrone | Springer

Regioselective Acetoxylation of Terminal Olefins Using a Palladium(II)–Thiadiazole Catalyst - Li - 2019 - European Journal of Organic Chemistry - Wiley Online Library

Palladium in biological media: Can the synthetic chemist's most versatile transition metal become a powerful biological tool? - ScienceDirect

Recent Progress in Palladium‐Catalyzed Radical Reactions - Sun - 2021 - Advanced Synthesis & Catalysis - Wiley Online Library
![Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C-S Bond Formation and Palladium-Catalyzed Intramolecular Arene-Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes ... Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C-S Bond Formation and Palladium-Catalyzed Intramolecular Arene-Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes ...](https://d3i71xaburhd42.cloudfront.net/95cff7feec2fffaff4d5ae744c1d00c294992095/2-Table1-1.png)
Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C-S Bond Formation and Palladium-Catalyzed Intramolecular Arene-Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes ...
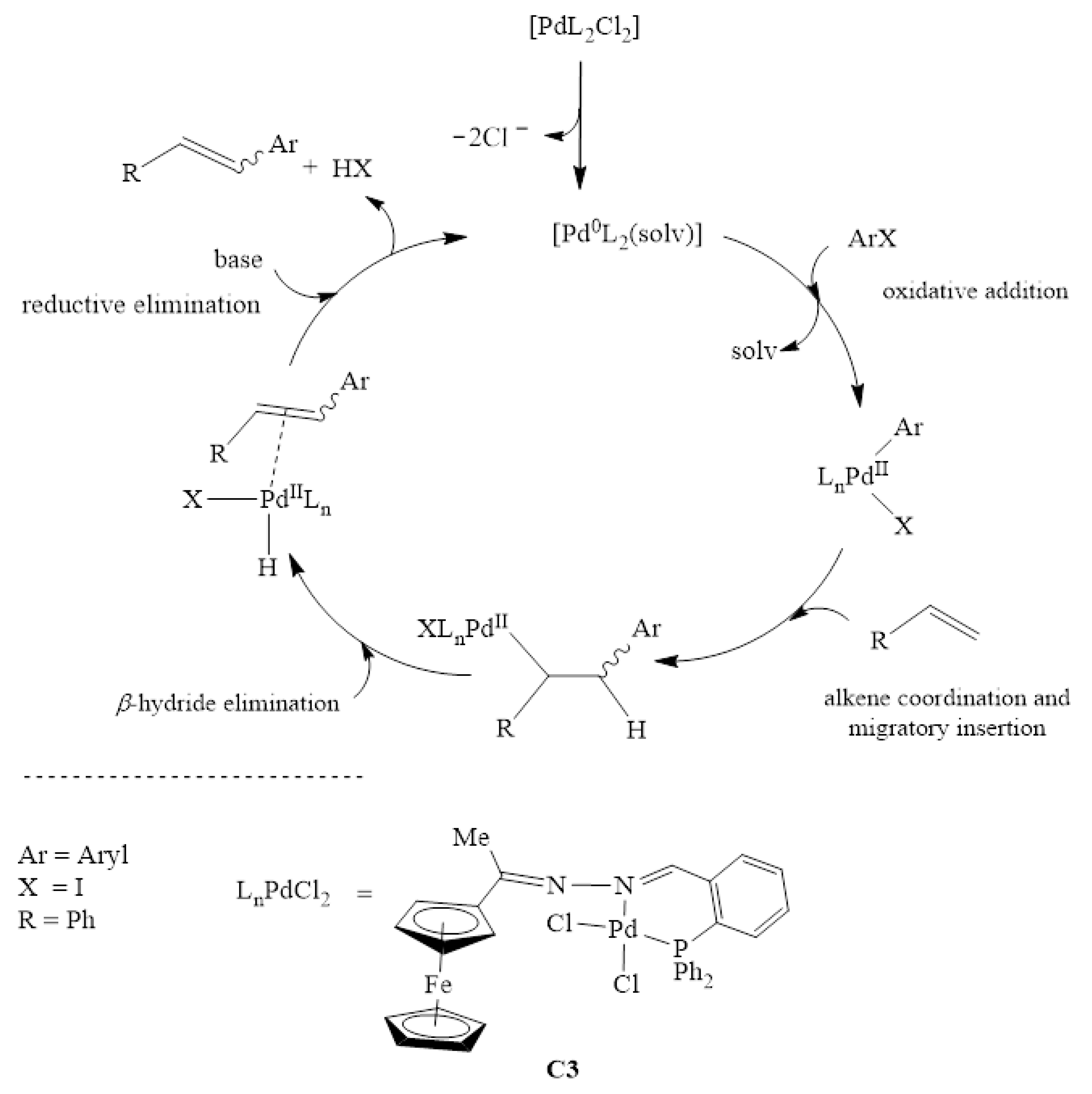
Catalysts | Free Full-Text | Ferrocenylimine Palladium (II) Complexes: Synthesis, Characterization and Application in Mizoroki-Heck and Suzuki-Miyaura Cross-Coupling Reactions | HTML
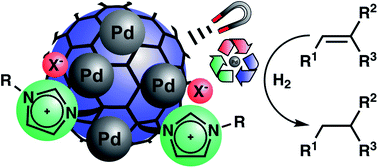
Palladium nanoparticles supported on ionic liquid modified, magnetic nanobeads – recyclable, high-capacity catalysts for alkene hydrogenation - RSC Advances (RSC Publishing)
![Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo[e]indoles - ScienceDirect Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo[e]indoles - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402017303848-fx1.jpg)
Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo[e]indoles - ScienceDirect

Palladium in biological media: Can the synthetic chemist's most versatile transition metal become a powerful biological tool? - ScienceDirect
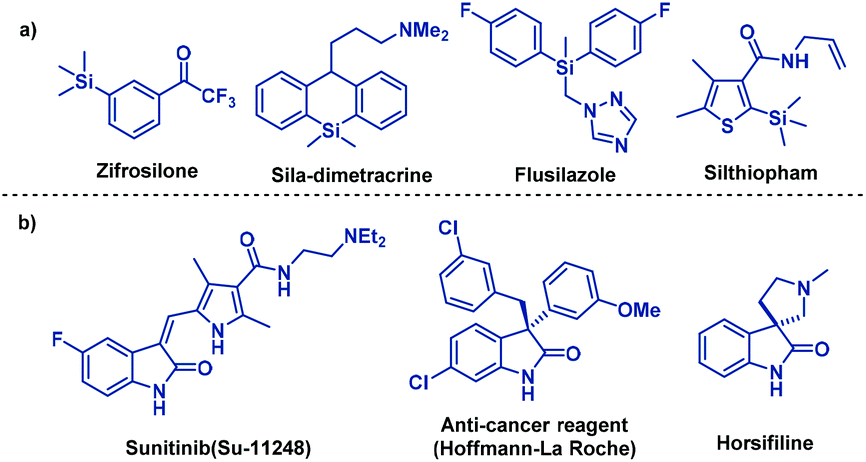
Palladium-catalyzed domino Heck-disilylation and Heck-monosilylation of alkene-tethered carbamoyl chlorides: synthesis of versatile silylated oxindoles - Organic Chemistry Frontiers (RSC Publishing)

Scheme 1 Synthesis of TTZ-1 and schematic of its fluorescent response... | Download Scientific Diagram
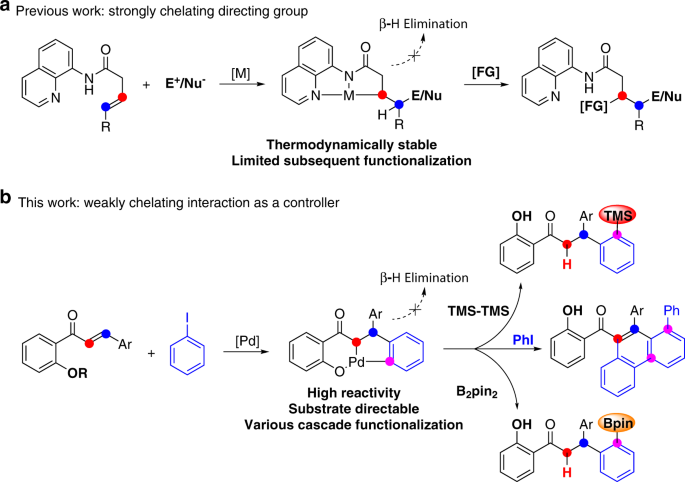
Quadruple C-H activation coupled to hydrofunctionalization and C-H silylation/borylation enabled by weakly coordinated palladium catalyst | Nature Communications

Recent Progress in Palladium‐Catalyzed Radical Reactions - Sun - 2021 - Advanced Synthesis & Catalysis - Wiley Online Library
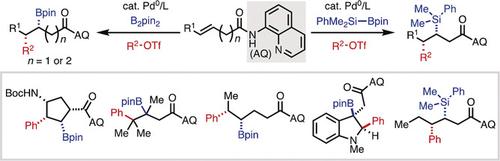
Palladium(0)-Catalyzed Directed syn-1,2-Carboboration and -Silylation: Alkene Scope, Applications in Dearomatization, and Stereocontrol by a Chiral Auxiliary.,Angewandte Chemie International Edition - X-MOL





